
The FDA recently issued guidance on the definition of Suspect and Illegitimate Products, set forth in the Federal Food Drug and Cosmetic Act1. This guidance aims to assist trading partners in putting systems in place to identify and handle suspect and illegitimate products, meeting Drug Supply Chain Security Act (DSCSA)2 requirements.
Trading partners are required to take action if they encounter suspect or illegitimate product.
Find a summary of the recent guidance below:
As defined in DSCSA upon its signing in 2013
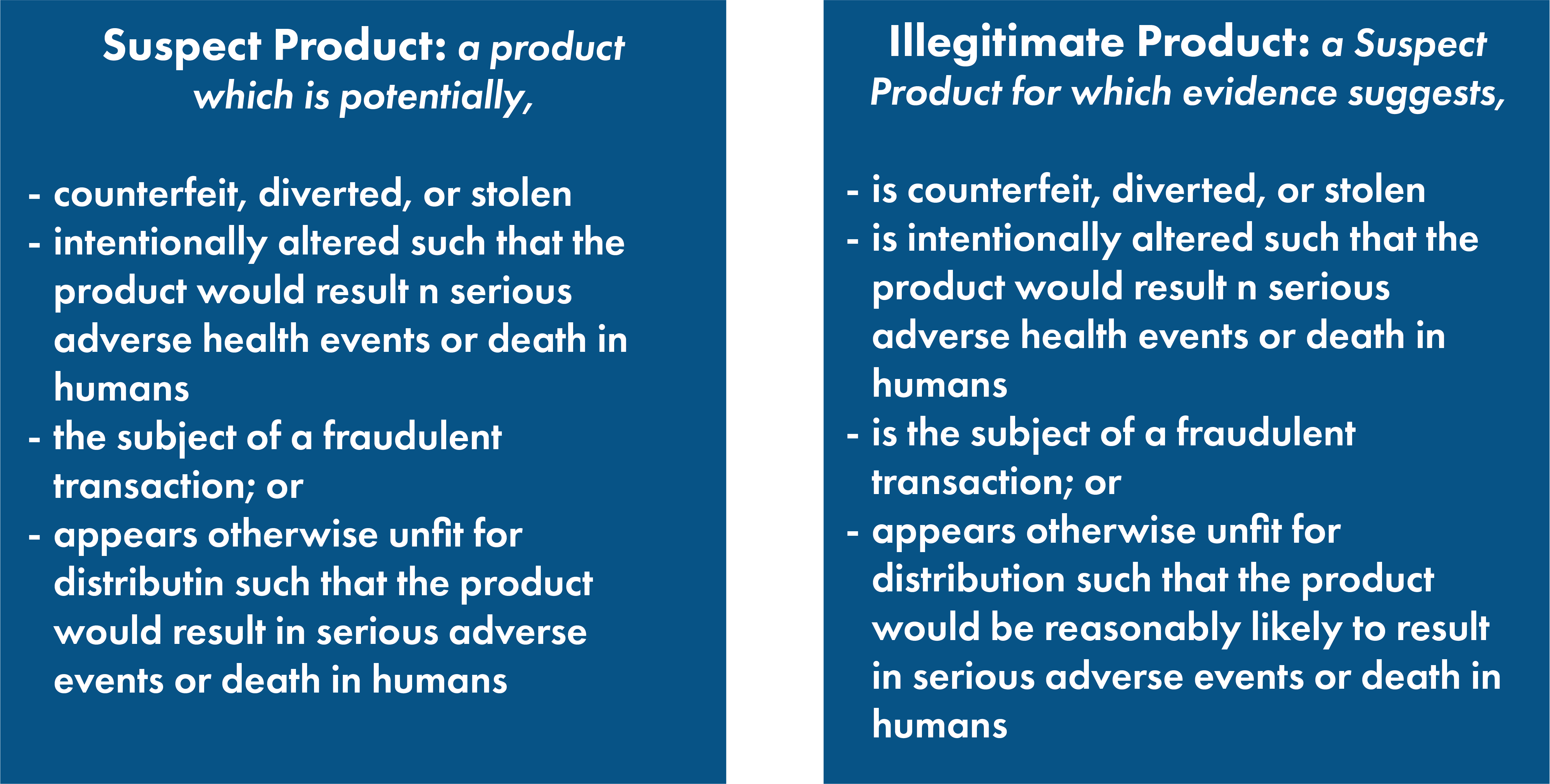
DSCSA requires trading partners to identify suspect product that enters its supply chain and have the ability to determine whether that product is illegitimate. According to the FDA, trading partners should focus on identifying products that are, or may be: counterfeit, diverted, stolen, intentionally adulterated, unfit for distribution, or the subject of a fraudulent transaction.
See clarifications on these definitions below.
Counterfeit – a drug, container, or drug label, which bears an unauthorized trademark, trade name, identifying mark, or any likeness thereof, of a manufacturer, processor, or distributor, other than the actual manufacturer, processor, or distributor, of such drug, thereby falsely representing itself as a product of the manufacturer, processor, or distributor.
Diverted – a product that leaves, and is then reintroduced, into the US Pharmaceutical Supply Chain; product that is for sale in a non-US market, which is then introduced into the US Supply Chain. Exceptions include: products that are authorized in response to FDA action (i.e, drug shortages), products used in the event of Emergency Use Authorization, products obtained through surveillance activities.
Stolen – Any product, packaging of a product, or prescription drug, that has been taken or removed without permissions of the owner of such product, or that is missing any or all portions of the drug as a result of being taken or removed without the permission of the owner. This includes packages, down to individual pills/capsules.
Consult the guidance: Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification, for guidance on identification of suspect and illegitimate product. - [Link]
Fraudulent Transaction – A transaction in which the [transaction] information, history, or statement contains information that was intentionally falsified by a trading partner.
Note: The FDA is aware that data errors, or other circumstances, may yield inaccurate transaction information where the data was not knowingly falsified. This is why trading partners must take steps to investigate all occurrences, to determine if a product is illegitimate.
Unfit for Distribution – a prescription drug that is nonsaleable because its sale would be violate the FD&C Act, and there is reason to believe its use would reasonably result in serious adverse events or death in humans.
Products are often deemed unfit due to doubts of the products safety, identity, strength quality or purity, to the extent that use of the product may reasonably result in serious adverse health events or death in humans.
The FDA recognizes that non-salable products are often removed from the supply chain without triggering investigation (expired drugs, reverse distribution). Only non-salable products that may otherwise reach a patient should be treated as suspect or illegitimate. However, these products should still be investigated should they meet any additional criteria outlined above.
To access the full FDA guidance, click here: [Link]
* * *
Thanks to Verify on Receipt and DSCSA Compliance as a Service, ConsortiEX is the industry leading DQSA Compliance provider.
Verify on Receipt™ - the premier DSCSA compliance solution from ConsortiEX, as an industry best practice, Verify on Receipt use of barcode scanning technology optimizes your workflows, accomplishing more than meeting compliance standards.
DSCSA Compliance as a Service - The solution that made ConsortiEX what it is today. DSCSA Compliance as a Service makes your compliance, our compliance
Assure-Trak® Compounding Management System - The only IV Workflow Management System currently meeting the needs of 503B Outsourcing Facilities and Health System Pharmacies
If you have any concerns about your compliance, or anything else, contact us today!
1 - Suspect: Section 581(21) of the FD&C Act)(21 U.S.C.360eee(21)
2- The Drug Supply Chain Security Act amended the FD&C to establish requirements for certain drug products in the United States
